 Hip Innovation Technology is betting on its Reverse HRS Hip to change the practice of total hip replacement
Hip Innovation Technology is betting on its Reverse HRS Hip to change the practice of total hip replacement
Most recent innovations in hip replacement have focused on the use of new materials, new surgical approaches and new assistive technologies. However, one company, Hip Innovation Technology* (HIT), is betting on its investment to change the practice of total hip replacement with an innovative design resulting in the world’s first reverse hip system. The Company is getting even closer to realizing a payoff on this bet by receiving US FDA Investigational Device Exemption (IDE) approval in January 2022 to initiate a pivotal multi-center clinical trial to evaluate the HIT Reverse Hip Replacement System (Reverse HRS)*. Most likely, HIT will be ready to submit for FDA approval of the device following the primary completion of the IDE trial slated for July 2026.
Who is Hip Innovation Technology?
Located in Boca Raton, Florida, Hip Innovation Technology is a privately held company formed in 2011 with the vision of developing and marketing orthopedic device solutions. The Company got its start through a seed round of funding with an initial raise of $225K. George Diamantoni, chief executive officer, is also the founder and CEO of Joint Innovation Technology, HIT’s parent company. He has over 25 years of experience in the healthcare industry including positions at The Upjohn Company, Pharmacia Corporation, Pfize, and Schering Plough. Zafer Termanini, MD, FACS, the inventor of the Interlocking Reverse Hip, serves as the president and chief medical officer of HIT. Termanini, an orthopedic surgeon with over 30 years of experience, is also a Diplomate of the American College of Disability Analysts and the American College of Forensic Examiners, with specialties in Orthopedic and Joint Reconstruction, Sports Medicine and Biomechanical Analysis.
What is a Reverse Hip Replacement?
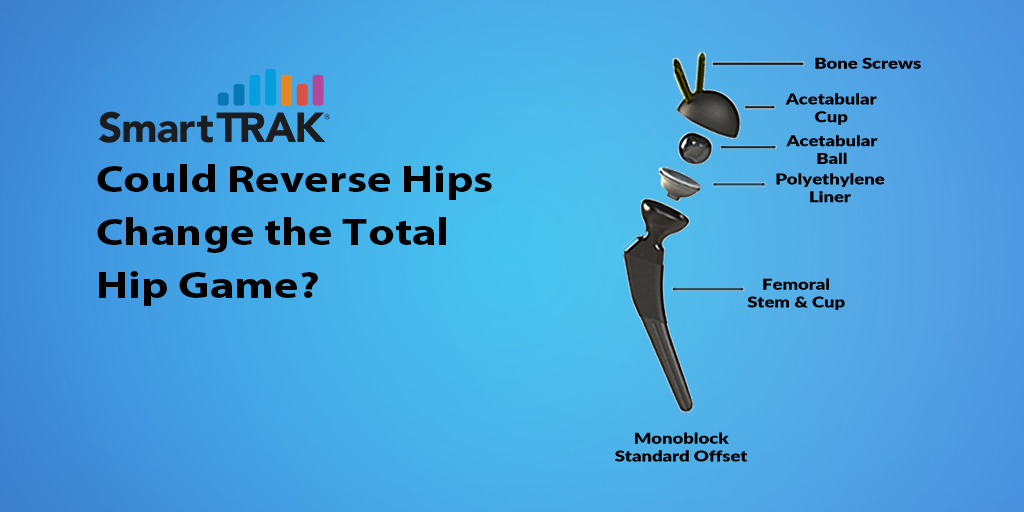
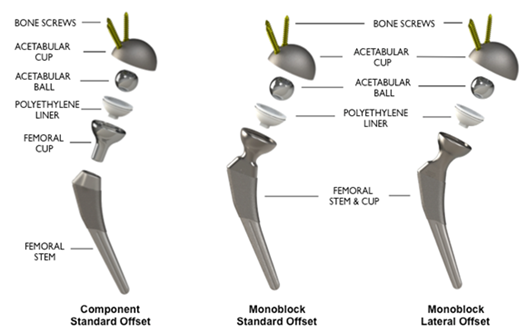
HIT Reverse HRS reverses the conventional geometry of hip replacement components. With the HIT Reverse HRS, the polyethylene-bearing surface is located on top of the femoral stem and the articulating ball of the joint is seated within the acetabular cup. The current Reverse HRS system consists of either a modular femoral stem and bearing cup or a monoblock stem and bearing cup with a highly crosslinked UHMWPE liner on the femoral side and, on the acetabular side, a metal cup with bone fixation screws and a cobalt-chrome acetabular ball that is seated on a trunnion inside the cup (Figure 1).
Figure 1: HIT Reverse HRS Hip Replacement System
Why a Reverse Hip? What Problems Does the Design Address?
Similar to French surgeon Dr. Paul Grammont’s creation of the reverse shoulder to address shoulder instability due to massive rotator cuff tears, HIT created the HRS Reverse Hip to address the need for increased hip stability. HIT is developing the hip system to address several complications following hip replacement including dislocation and instability, component positioning and edge loading. According to HIT, the Reverse HRS design allows the edge of the femoral cup to interlock in the space between the acetabular ball and the acetabular cup minimizing the risk of dislocation at extended ranges of motion. Also, HRS claims that the center of rotation of the Reverse HRS compares to a normal natural hip or a well-positioned total hip replacement. In addition, the Company believes the reverse design reduces high contact stresses across the hip joint, distributes wear evenly across the bearing surface and minimizes edge loading.
Just How Big are these Problems Anyway?
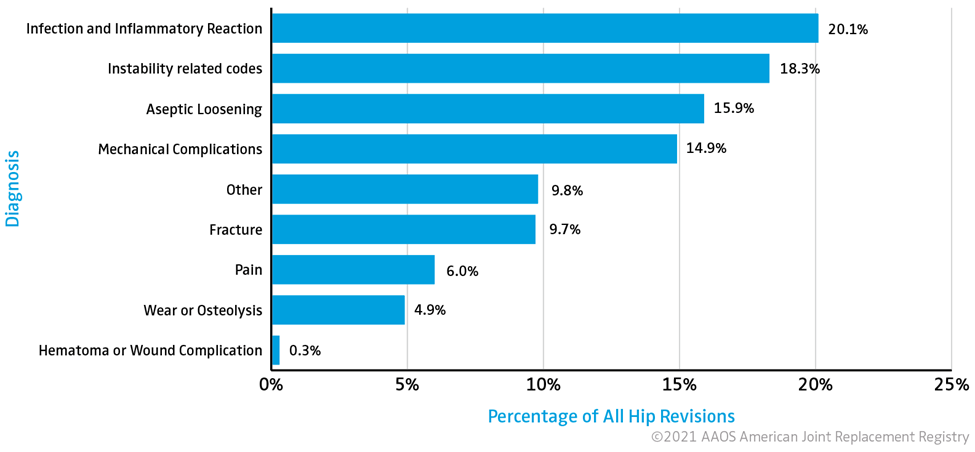
The American Joint Replacement Registry reported in its 2021 annual report that the number two diagnosis leading to hip replacement revisions was postop instability in 18.3% of cases (Figure 2). Aseptic loosening, mechanical complications and wear or osteolysis, which can be a result of edge loading of the primary implant, comprised 35.7% of hip revisions tracked by the registry through 2020. Combined these complications are associated with approximately 54% of hip revisions.
Figure 2: Distribution of Diagnosis Associated with All Hip Revisions Reported by AJRR, 2012-2020
What Have the Results Been so Far?
Prior to in vivo studies, HIT conducted bench-level tests of the Reverse HRS demonstrating that regardless of acetabular cup positioning, the poly cup bearing wear was consistent with no runaway wear or edge loading. The Company progressed to cadaver research that showed in both normal and extreme mal-positioned acetabular cup positions, the Reverse HRS was stable with no impingement or dislocations. Then, in July 2022, HIT announced a poster presentation at the Canadian Orthopaedic Association annual meeting reporting Phase 1 data showing radiographic stability of the Reverse HRS hip implanted in 22 patients. The Phase 1 data suggested a low risk of aseptic loosening and showed significant improvements in patient-reported outcomes measures at 24-month follow-up.
So What’s the Buzz?
The HIT Reverse HRS Hip has received mixed reviews on social media. Many surgeons commenting on Twitter expressed skepticism regarding the need for a reverse hip or if it would even work while others were more open to the new concept. Dr. Naan Derthaal’s comment on a Twitter announcement about the Reverse HRS IDE study summed up the general sentiment about the technology. “Well, we solved the problem that we didn’t have.”
SmartTRAK believes HIT may be facing high odds against the commercialization of the Reverse HRS based on the difficulty of gaining FDA approval for a new technology and current surgeon sentiment. The Company may need to partner with a larger Orthopedic Market player with pockets deep enough to push FDA approval, secure payer reimbursement and develop a robust marketing and surgeon education campaign to drive the initial adoption of this technology.
*These links can only be viewed by SmartTRAK subscribers. For more information on SmartTRAK, including how to receive a demo and subscribe, please click the button below.
SmartTRAK is a comprehensive, easy-to-use, business intelligence solution for the Life Sciences Industry that provides breaking updates on pertinent company news, potential treatments and guidance, international survey results, statistical analysis and impact by country, studies, trial results, financial impact and more. All subscribers receive a Daily Updates email containing the latest need-to-know news curated by our expert analysts. It's the perfect way to start your day! If you would like to learn more about SmartTRAK, just here.





